RESEARCH
The major research goals of the Hansen lab are to enhance the synthetic pharmacology of ionotropic glutamate receptors, which are involved in a multitude of disorders in the central nervous system, and improve our knowledge of their physiological roles in normal brain function and in neuropathological conditions. Our research includes structural and mechanistic questions in neurophysiology and neuropharmacology as well as efforts to identify and validate novel strategies and new glutamate receptor targets that can be exploited for therapeutic intervention.
NMDA-type glutamate receptors are ligand-gated ion channels that mediate excitatory neurotransmission in the central nervous system, but are also implicated in many neurological and psychiatric disorders. The molecular pharmacology and structure-function relationship of NMDA receptors is an important focus of our research, and we have identified new ligands from assay development to compound screening and characterizated of novel modulators and their mechanisms of action. This effort has led to the discovery of multiple novel classes of small-molecule modulators of NMDA receptor function and new modulatory ligand binding sites. These newly discovered modulatory sites can be exploited for the development of subunit-selective pharmacological tools and create new avenues for drug discovery.
Development of novel subunit-selective NMDA receptor ligands
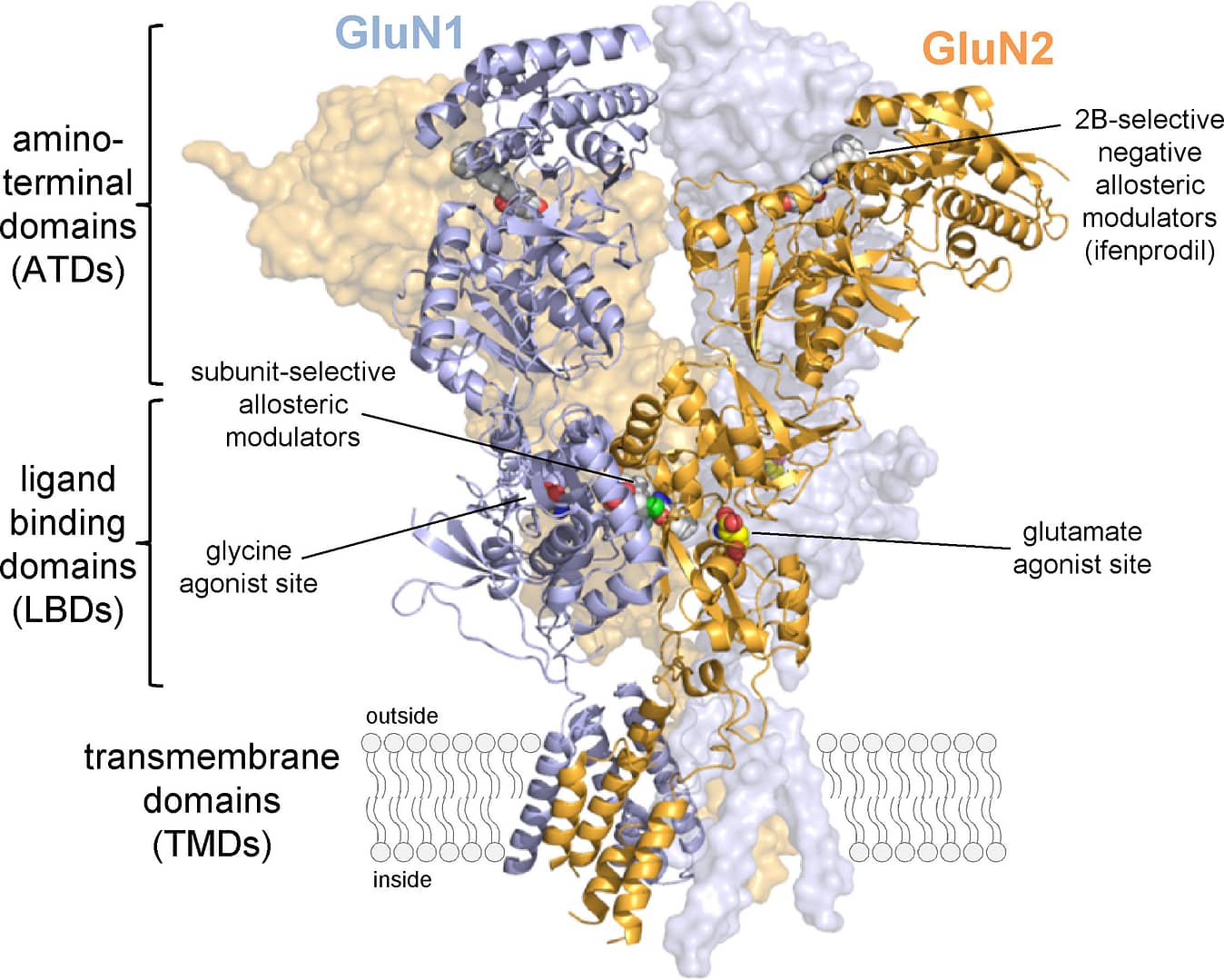
NMDA receptors are assembled from multiple different subunits with distinct localization in the brain, thereby creating considerable diversity in NMDA receptor subtypes among brain regions and during brain development. However, relatively few subunit-selective positive and negative modulators have been developed, and this lack of useful pharmacological tools is hampering our understanding of the physiological roles of NMDA receptor subtypes. Closing the gap regarding the lack of subunit-selective NMDA receptor modulators is one of our main goals, and we have made remarkable progress. In addition, we make efforts to elucidate the structural determinants and mechanisms of action for new subunit-selective modulators, competitive antagonists, and agonists.
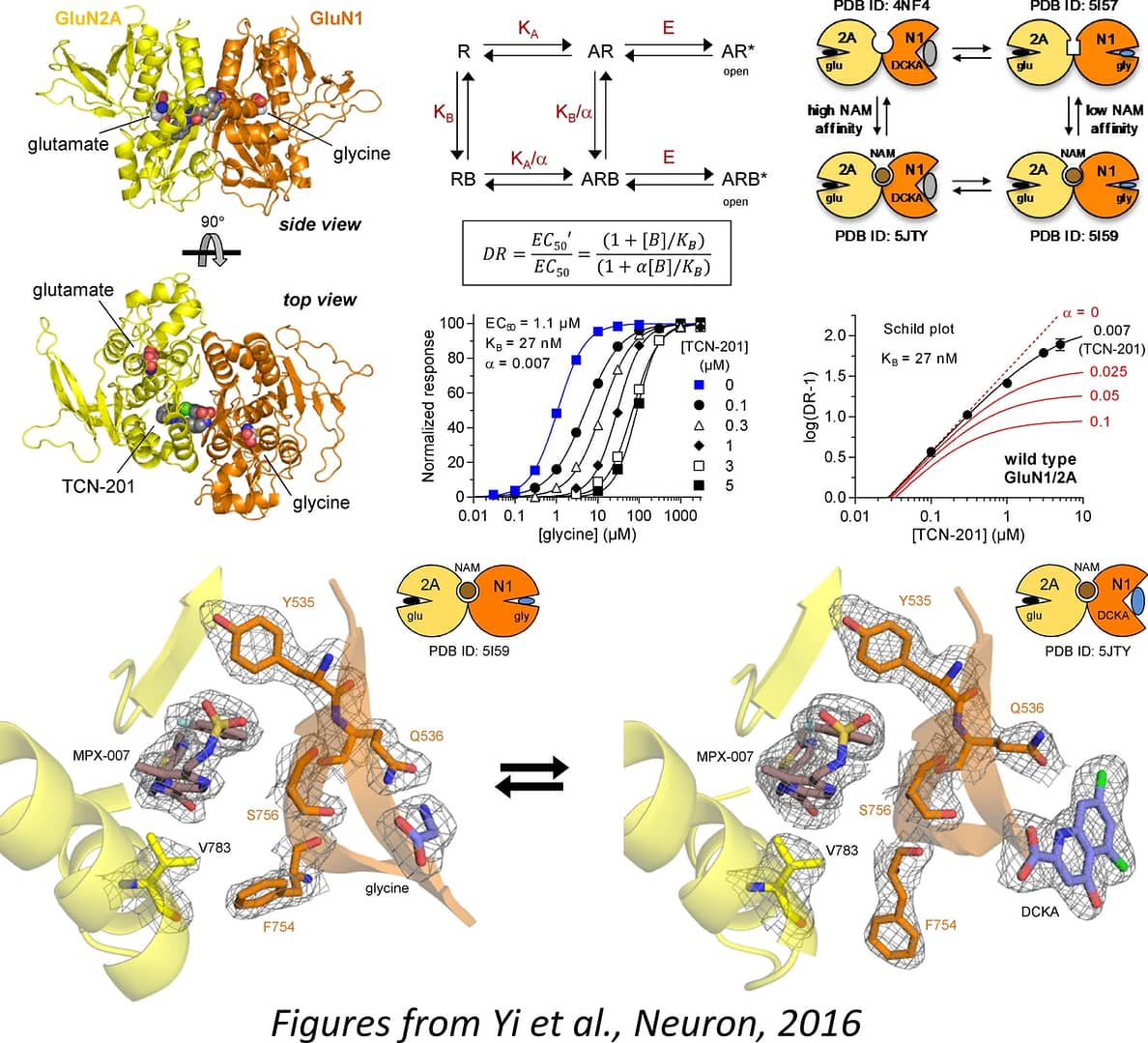

Pharmacology and function of triheteromeric NMDA receptors

The seven NMDA receptor subunits (GluN1, GluN2A-D, and GluN3A-B) assemble as tetrameric receptors composed of two GluN1 together with two GluN2 or two GluN3 subunits. Most, if not all, NMDA receptor-expressing cells express two different GluN2 subunits (e.g. GluN2A and GluN2B), and the majority of native NMDA receptors are triheteromers that contain two GluN1 together with two different GluN2 subunits (e.g. GluN1/GluN2A/GluN2B). Function and pharmacology of these triheteromeric NMDA receptors are poorly understood due to the problem that co-expression of three different subunits in heterologous expression systems will yield multiple different receptor populations. We have developed a method to control the subunit composition of NMDA receptors, thereby enabling selective expression of triheteromeric NMDA receptors. This approach provides opportunities to develop therapeutic agents that target disease-relevant native triheteromeric NMDA receptor sutypes.


Structure, function, and physiology of GluN3-containing NMDA receptors


Many basic questions related to the physiological roles of GluN3-containing NMDA receptors are unanswered. Despite the lack of basic understanding, we know from studies using GluN3-deficient and GluN3-overexpressing mice that GluN3 subunits are involved in synapse maturation, synaptic plasticity, and neuroprotection. The GluN3 NMDA receptor subunits could therefore be promising new therapeutic targets in a number of brain disorders.

Supported by:


