NEWS
Paper accepted in the Journal of General Physiology
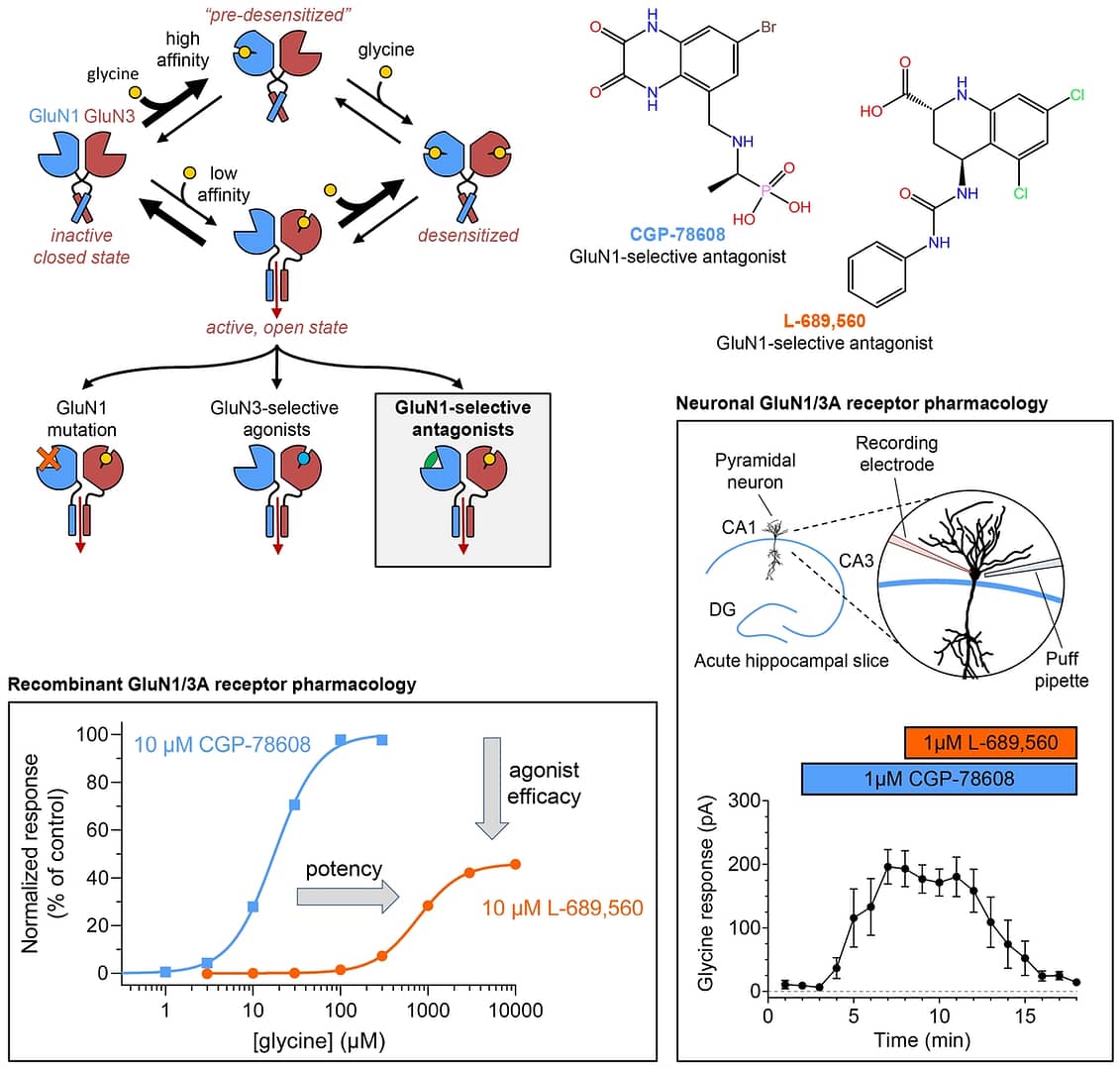
Congratulations to Nirvan Rouzbeh and co-authors on the new paper in the Journal of General Physiology. This study demonstrates that robust potentiation of GluN1/3 receptors by CGP-78608 is facilitated by positive intra-subunit allosteric interactions that increase glycine potency and efficacy at the GluN3 subunit. By contrast, binding of L-689,560 to the GluN1 agonist binding domain (ABD) reduces glycine potency and efficacy at the GluN3 subunit. Molecular dynamics simulations, some of which can be seen here, suggest that more open conformations of the GluN1 ABD, such as those selected by CGP-78608, promote glycine binding to GluN3A, whereas more closed GluN1 ABD conformations, like those selected by L-689,560, negatively modulate glycine binding. Thus, the pharmacology of GluN1/3 receptors evaluated in functional assays is highly dependent on the GluN1-selective competitive antagonists or GluN1 agonist binding site mutations that are utilized to prevent receptor desensitization. The intra-subunit allosteric interactions described here therefore highlight a complex structure-function relationship that impacts the development of potential therapeutic agents and pharmacological tool compounds for native GluN1/3 NMDA receptors.
3/29/2023
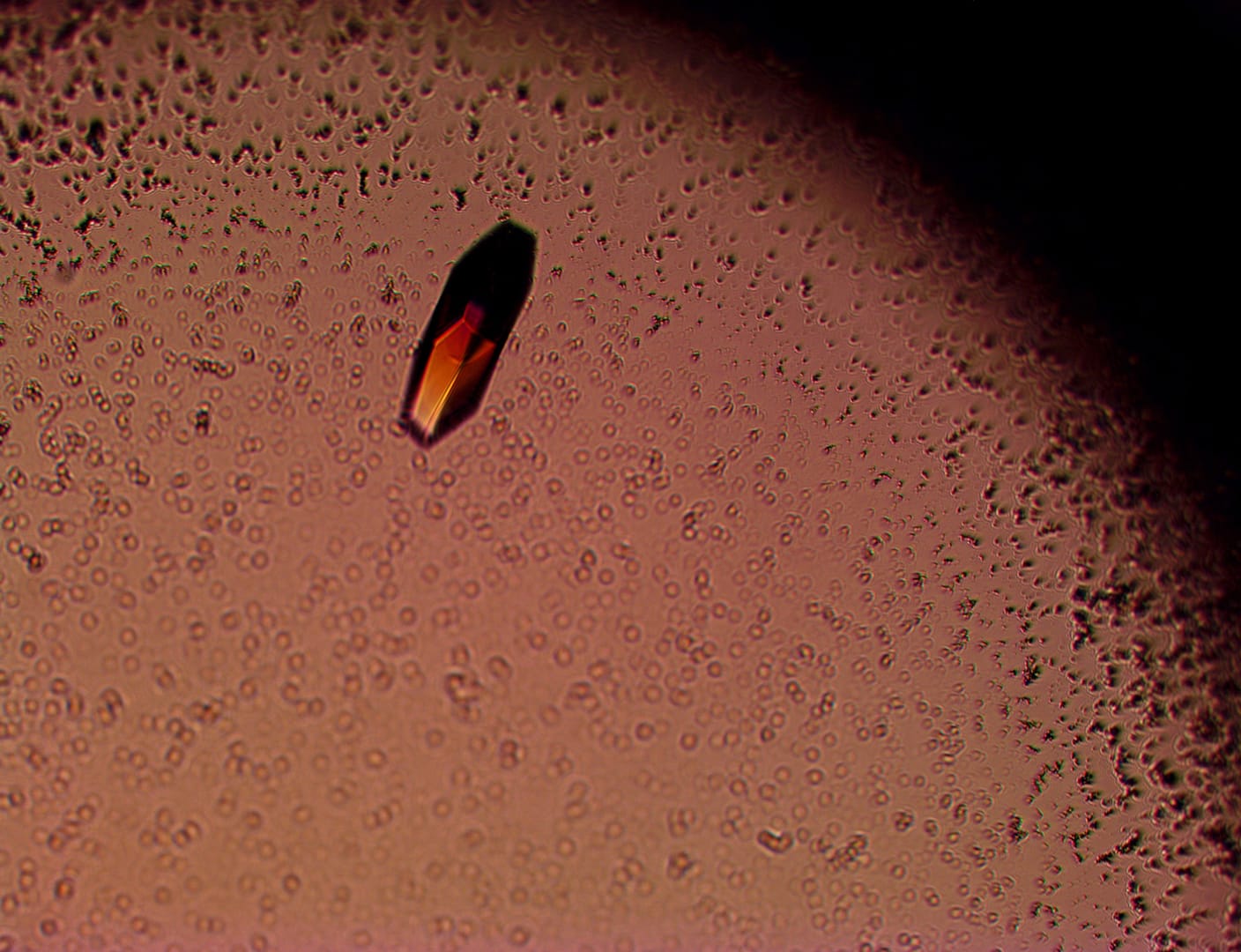
First of many protein crystals
Chris discovered his first protein crystal today. Hopefully, the first of many and exciting times lie ahead.
3/27/2023
Paper accepted in Frontiers in Chemistry
Congratulations to Fabao Zhao and co-authors on the manuscript accepted in Frontiers in Chemistry. The study describes the design and pharmacological evaluation of a novel series of NMDA receptor glycine site agonists that bind GluN1, but display GluN2 subunit-specific variation in activity among NMDA receptor subtypes. The study provides insights to agonist binding at the GluN1 subunit of NMDA receptors and provide new opportunities for the design of glycine site agonists.
10/19/2022
